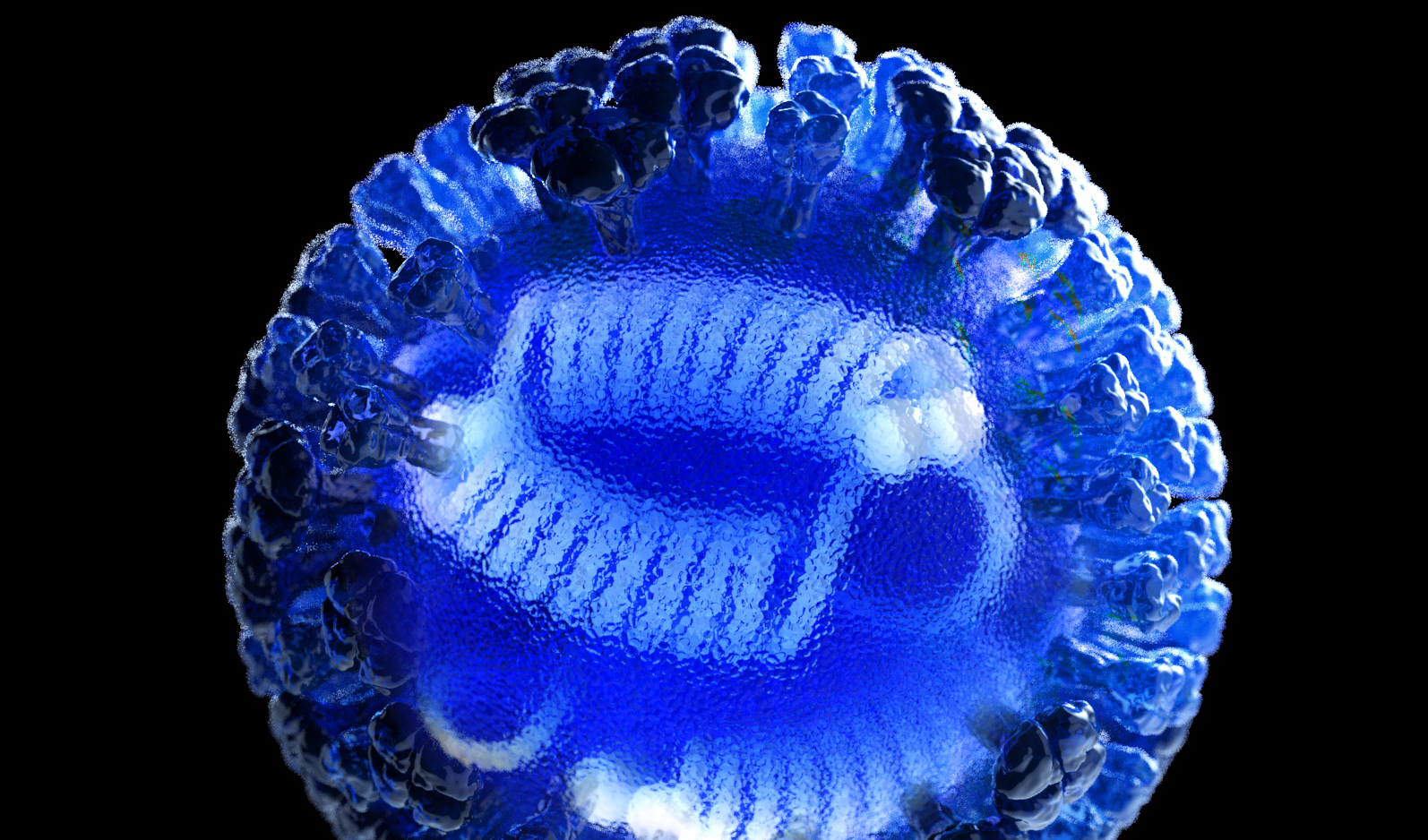
A multidisciplinary team led by Georgia Institute of Technology (Georgia Tech) researchers has received $14.7 million in funding from the Defense Advanced Research Projects Agency (DARPA) to develop novel diagnostic devices able to rapidly identify the bacteria causing sepsis – and viruses that cause respiratory infections such as RSV, SARS-CoV-2, and influenza.
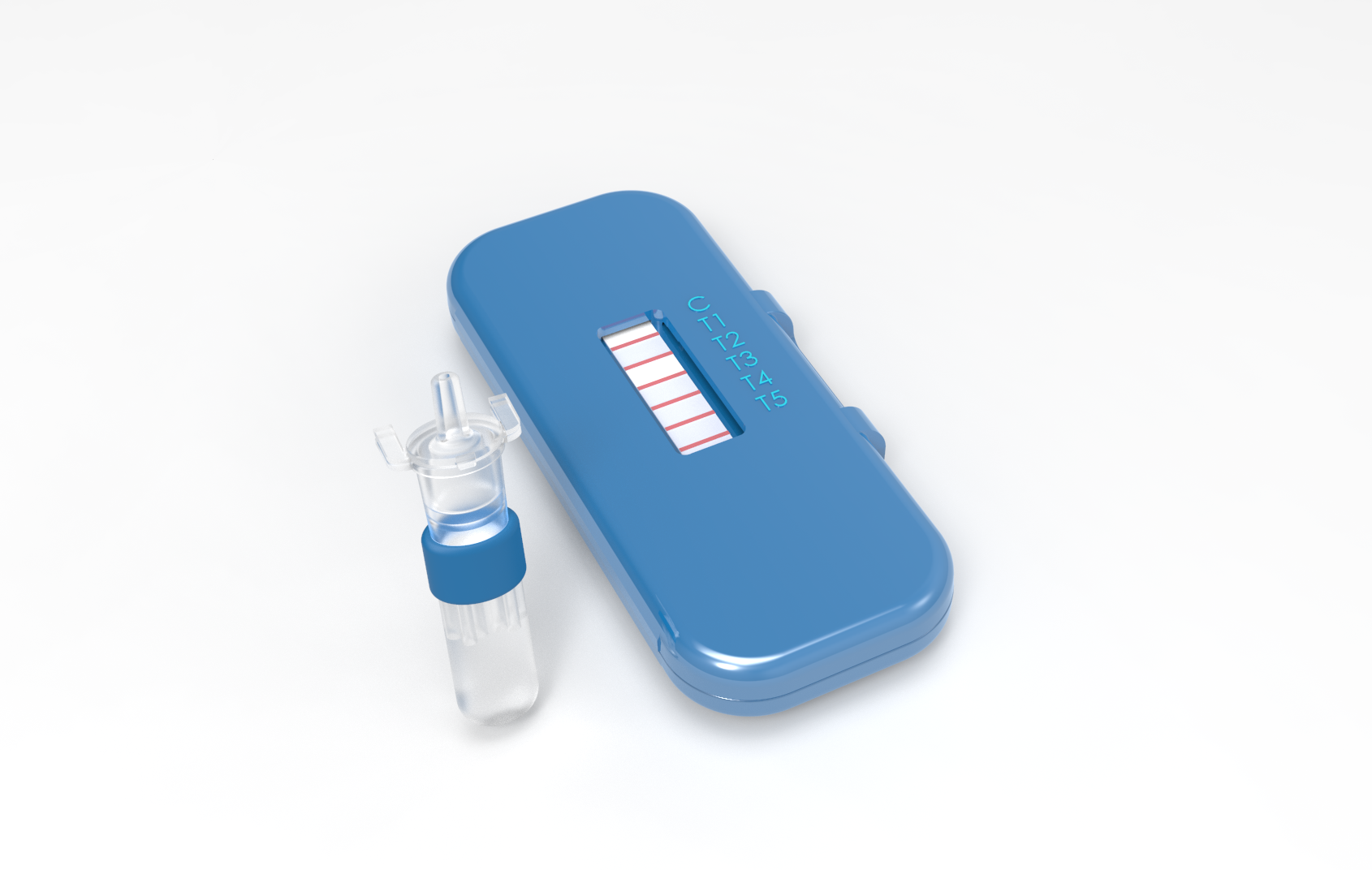
The novel nucleic acid detection devices will use the CRISPR Cas13a enzyme to initiate a synthetic biology workflow that will lead to the production of a visible signal if a targeted infectious agent is present in a sample of blood – or fluid from a nasal or throat swab. The devices will be simple to use, similar to the lateral-flow technology in home pregnancy tests. The devices will provide diagnostic capabilities to low-resource areas such as clinics and battlefield medical units, allowing treatment of infections to begin more quickly – potentially saving lives.
“This new technology will make it much faster and more cost-effective to diagnose these infections,” said Mike Farrell, a Georgia Tech Research Institute (GTRI) principal research scientist who is leading the project. “You would obtain a sample, put it into a device, diagnose the underlying pathogen, and be able to provide a treatment. This could be a huge leap forward in rapidly diagnosing these diseases where sophisticated laboratory testing isn’t available.”
Funded by DARPA’s Detect It with Gene Editing Technologies (DIGET) program, the project – known as Tactical Rapid Pathogen Identification and Diagnostic Ensemble (TRIAgE) – also includes researchers from Emory University and two private sector companies. The goal will be to detect 10 different pathogens with each device.
Detection Reaction Begins with CRISPR Cas13a Enzyme
Detection of a pathogen will begin with exposure of a patient sample to the CRISPR Cas13a enzyme with guide proteins containing RNA genetic sequences from the targeted pathogens. If a genetic sequence in the device matches a sequence in the patient sample, the enzyme will begin breaking down the targeted RNA.
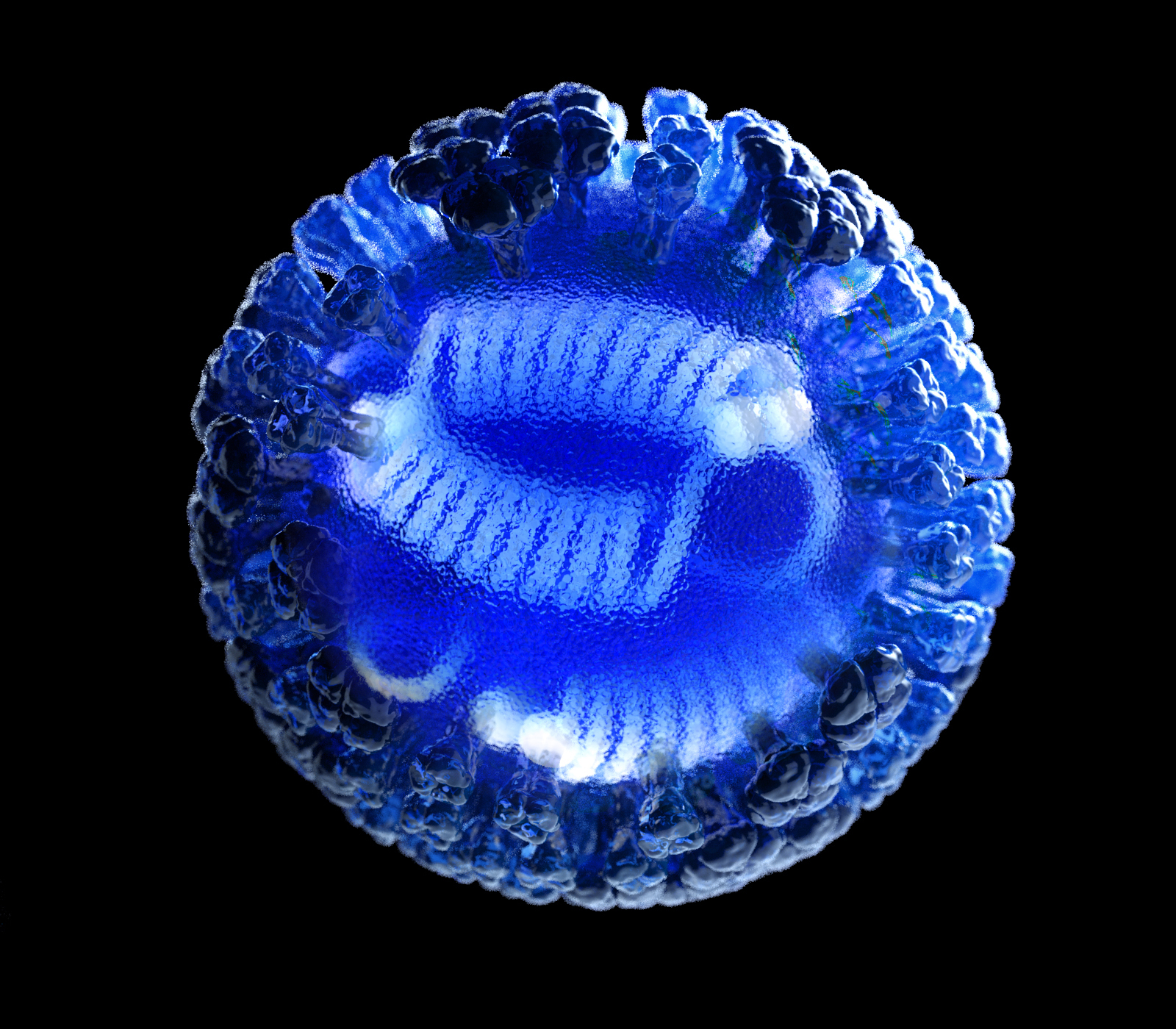
Development of the CRISPR Cas13a component of the project will be led by Phil Santangelo, a professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University and one of the team’s collaborators. CRISPR Cas13a differs from Cas9 technology, which has become known for its ability to edit DNA, which Cas13A will not do.
Once the Cas13a enzyme breaks down the pathogen RNA, that will trigger additional reactions to amplify the signal and create a visible blue line in the device within 15 minutes.
Synthetic Biology Workflow Signals Pathogen Presence
“We will be assembling a synthetic biology workflow that takes an initial signal created by CRISPR-based nucleic acid detection and amplifies it using the same cell-free synthetic biology approaches we have used to create sensors for detecting small molecules and metals: turning on genes that create a visual readout so that expensive instruments, and even electricity, are unnecessary,” explained Mark Styczynski, a professor in Georgia Tech’s School of Chemical and Biomolecular Engineering and another team collaborator.
“As part of the DIGET project, we will be leveraging my group’s expertise in minimal-equipment diagnostics,” he added. “The biological ‘parts’ we develop can be reused to transduce signals for the detection of essentially any nucleic acid sequence.”
Another Georgia Tech researcher, King Jordan, professor and director of the Bioinformatics Graduate Program in the School of Biological Sciences, will mine the genomes of the targeted pathogens for optimal Cas13a target sequences as well as the corresponding Cas13a RNA guide sequences.
Devices Must be Both Sensitive and Specific
Beyond specifically identifying the pathogen or pathogens causing an infection, the diagnostic devices being developed must also be very sensitive – able to detect as few as 10 copies of the target pathogen in a sample. “A major technological challenge is achieving the level of signal amplification within the device’s synthetic biology circuit to reach the needed level of sensitivity,” Farrell said.
The ability to detect 10 different pathogens with a single lateral-flow assay is an ambitious goal for a device that depends on a synthetic biology circuit and is designed for use in the field, he added. Lateral-flow assays commonly used in home or point-of-care medical tests operate by applying a liquid sample to a pad containing reactive molecules. The molecules may create visible positive or negative reactions, depending on the design.
“You just put the sample on the device and it does its thing,” Farrell said. “If the target pathogen is present, a line turns blue and you can see it with your eye.”
Early Diagnosis Can be Life-Saving
Sepsis is an infection of the bloodstream by any of a number of different bacteria. These bacteria can originate from a lower respiratory infection, kidney or bladder infection, digestive system breakdown, catheter site, wound, or burn. Sepsis results in a severe and persistent inflammatory response that can lead to disrupted blood flow, tissue damage, organ failure, and death.
“It’s important to identify the specific bacteria causing the sepsis because that informs the type of antimicrobial therapy that’s needed,” said Farrell. “The sooner you can identify the underlying pathogen, the faster you can provide the proper medical care, and the more likely it is that the patient will survive. Current laboratory-based diagnostic methods can take between 24 and 72 hours, and that is just too long.”
Improving diagnostics for sepsis and respiratory diseases will have applications to both the military and civilian worlds, particularly in locations without easy access to laboratory testing.
“Wounded soldiers in the field are very susceptible to sepsis blood infections, and common respiratory diseases can affect troop readiness, so from a military standpoint, having this rapid diagnostic test would be very significant,” Farrell said. “In low-resource environments, being able to diagnose these diseases with a single test would be huge as well. Being able to identify the underlying bacteria behind sepsis more quickly could save a lot of lives.”
Beyond the university researchers, the project includes Global Access Diagnostics, a manufacturer of lateral-flow devices, and Ginkgo Bioworks, which manufactures proteins essential to the diagnostics.
The five-phase project is expected to last for four years and will conclude with field validation and a transition to manufacturing. The devices will need to win FDA approval before they can be used, so there is a significant regulatory review aspect to the project, Farrell said.
Approved for Public Release, Distribution Unlimited
Writer: John Toon (john.toon@gtri.gatech.edu)
GTRI Communications
Georgia Tech Research Institute
Atlanta, Georgia
The Georgia Tech Research Institute (GTRI) is the nonprofit, applied research division of the Georgia Institute of Technology (Georgia Tech). Founded in 1934 as the Engineering Experiment Station, GTRI has grown to more than 2,900 employees, supporting eight laboratories in over 20 locations around the country and performing more than $800 million of problem-solving research annually for government and industry. GTRI's renowned researchers combine science, engineering, economics, policy, and technical expertise to solve complex problems for the U.S. federal government, state, and industry.



![For GTRI Senior Research Associate Margarita Gonzalez, who had a diverse professional career before coming to GTRI, “All [the things you have done] in your life leads to this moment.” (Photo credit: Sean McNeil)](/public/test/styles/news_listing/public/article/2021-10/Margarita%20Gonzalez_01.jpg?itok=QlpeHy8M)
